Chemistry Interactive Textbook 2.1.0
Free Version
Publisher Description
Chemistry Interactive Textbook - Chemistry TextBook TextBook by OpenStax plus MCQ, Essay Questions & Key Terms
Chemistry TextBook TextBook by OpenStax plus MCQ, Essay Questions & Key Terms
Chemistry is designed to meet the scope and sequence requirements of the two-semester general chemistry course. The textbook provides an important opportunity for students to learn the core concepts of chemistry and understand how those concepts apply to their lives and the world around them. The book also includes a number of innovative features, including interactive exercises and real-world applications, designed to enhance student learning.
* Complete Textbook by OpenStax
* Multiple Choices Questions (MCQ)
* Essay Questions Flash Cards
* Key-Terms Flash Cards
Powered by https://www.jobilize.com/
1. Essential Ideas
1.3. Physical and Chemical Properties
1.5. Measurement Uncertainty, Accuracy, and Precision
1.6. Mathematical Treatment of Measurement Results
2. Atoms, Molecules, and Ions
2.1. Early Ideas in Atomic Theory
2.2. Evolution of Atomic Theory
2.3. Atomic Structure and Symbolism
2.4. Chemical Formulas
2.5. The Periodic Table
2.6. Molecular and Ionic Compounds
2.7. Chemical Nomenclature
3. Composition of Substances and Solutions
3.1. Formula Mass and the Mole Concept
3.2. Determining Empirical and Molecular Formulas
3.3. Molarity
3.4. Other Units for Solution Concentrations
4. Stoichiometry of Chemical Reactions
4.1. Writing and Balancing Chemical Equations
4.2. Classifying Chemical Reactions
4.3. Reaction Stoichiometry
4.4. Reaction Yields
4.5. Quantitative Chemical Analysis
5. Thermochemistry
5.1. Energy Basics
5.2. Calorimetry
5.3. Enthalpy
6. Electronic Structure and Periodic Properties of Elements
6.1. Electromagnetic Energy
6.2. The Bohr Model
6.3. Development of Quantum Theory
6.4. Electronic Structure of Atoms (Electron Configurations)
6.5. Periodic Variations in Element Properties
7. Chemical Bonding and Molecular Geometry
7.1. Ionic Bonding
7.2. Covalent Bonding
7.3. Lewis Symbols and Structures
7.4. Formal Charges and Resonance
7.5. Strengths of Ionic and Covalent Bonds
7.6. Molecular Structure and Polarity
8. Advanced Theories of Covalent Bonding
8.1. Valence Bond Theory
8.2. Hybrid Atomic Orbitals
8.3. Multiple Bonds
8.4. Molecular Orbital Theory
9. Gases
9.1. Gas Pressure
9.2. Relating Pressure, Volume, Amount, and Temperature: The Ideal Gas Law
9.3. Stoichiometry of Gaseous Substances, Mixtures, and Reactions
9.4. Effusion and Diffusion of Gases
9.5. The Kinetic-Molecular Theory
9.6. Non-Ideal Gas Behavior
10. Liquids and Solids
10.1. Intermolecular Forces
10.2. Properties of Liquids
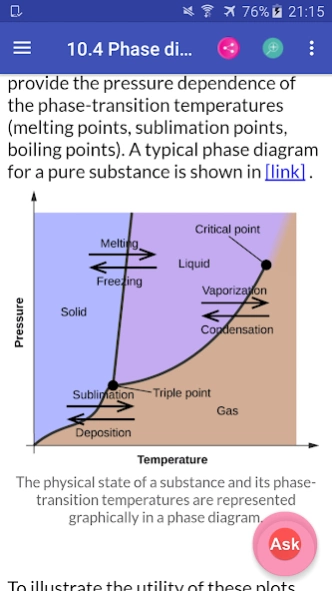
10.3. Phase Transitions
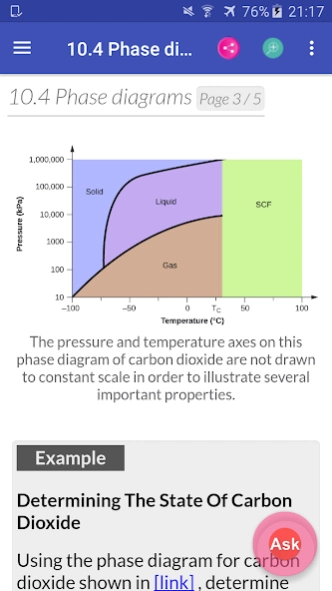
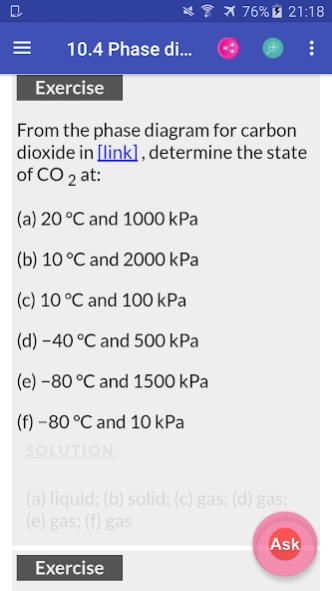
10.4. Phase Diagrams
10.5. The Solid State of Matter
10.6. Lattice Structures in Crystalline Solids
11. Solutions and Colloids
11.1. The Dissolution Process
11.2. Electrolytes
11.3. Solubility
11.4. Colligative Properties
12. Kinetics
12.1. Chemical Reaction Rates
12.2. Factors Affecting Reaction Rates
12.3. Rate Laws
12.4. Integrated Rate Laws
12.5. Collision Theory
12.6. Reaction Mechanisms
12.7. Catalysis
13. Fundamental Equilibrium Concepts
13.1. Opener
13.2. Chemical Equilibria
13.3. Equilibrium Constants
13.4. Shifting Equilibria: Le Châtelier’s Principle
13.5. Equilibrium Calculations
14. Acid-Base Equilibria
14.1. Brønsted-Lowry Acids and Bases
14.2. pH and pOH
14.3. Relative Strengths of Acids and Bases
14.4. Hydrolysis of Salt Solutions
14.5. Polyprotic Acids
14.6. Buffers
14.7. Acid-Base Titrations
15. Equilibria of Other Reaction Classes
15.1. Precipitation and Dissolution
15.2. Lewis Acids and Bases
15.3. Multiple Equilibria
16. Thermodynamics
16.1. Spontaneity
16.2. Entropy
16.3. The Second and Third Laws of Thermodynamics
16.4. Free Energy
17. Electrochemistry
18. Representative Metals, Metalloids, and Nonmetals
19. Transition Metals and Coordination Chemistry
20. Organic Chemistry
21. Nuclear Chemistry
About Chemistry Interactive Textbook
Chemistry Interactive Textbook is a free app for Android published in the Teaching & Training Tools list of apps, part of Education.
The company that develops Chemistry Interactive Textbook is QuizOver.com. The latest version released by its developer is 2.1.0.
To install Chemistry Interactive Textbook on your Android device, just click the green Continue To App button above to start the installation process. The app is listed on our website since 2018-03-18 and was downloaded 1 times. We have already checked if the download link is safe, however for your own protection we recommend that you scan the downloaded app with your antivirus. Your antivirus may detect the Chemistry Interactive Textbook as malware as malware if the download link to com.quizover.app.course.col11760 is broken.
How to install Chemistry Interactive Textbook on your Android device:
- Click on the Continue To App button on our website. This will redirect you to Google Play.
- Once the Chemistry Interactive Textbook is shown in the Google Play listing of your Android device, you can start its download and installation. Tap on the Install button located below the search bar and to the right of the app icon.
- A pop-up window with the permissions required by Chemistry Interactive Textbook will be shown. Click on Accept to continue the process.
- Chemistry Interactive Textbook will be downloaded onto your device, displaying a progress. Once the download completes, the installation will start and you'll get a notification after the installation is finished.